
Newsroom
A research team led by Prof. ZHU Ping from the Institute of Biophysics of the Chinese Academy of Sciences (CAS), along with collaborators from the Institute of Physics of CAS, has uncovered the intricate mechanism by which the NuA4 complex acetylates histones H2A.Z and H4, a process crucial for gene transcription regulation in yeast.
The study was published in PNAS on March 18.
NuA4 is the only essential acetyltransferase in yeast, responsible for acetylating multiple lysine residues on the N-terminal tails of histones H2A, H2A.Z, and H4. Despite its crucial role, the mechanisms by which histone acetyltransferases modify multiple lysine residues on different histones within a nucleosome have remained poorly understood—until now.
In this study, using advanced cryo-electron microscopy (cryo-EM) and single-molecule Förste resonance energy transfer (smFRET) techniques, the researchers resolved seven distinct structural states of piccolo NuA4 (pNuA4) bound to H2A.Z-containing nucleosomes. Their findings reveal that pNuA4 dynamically searches for its substrates, preferring H4 over H2A.Z for acetylation.
This study provides a complete process of the acetylation of nucleosome by pNuA4, and clarifies the specific mechanism of pNuA4 when acetylating multiple lysine substrates, and shows the dynamic sequence and structural model of pNuA4 during the acetylation of the nucleosome.
These findings help researchers to understand the mechanism of gene transcription regulation by histone acetylation, and provides new insights for the treatment of related diseases.
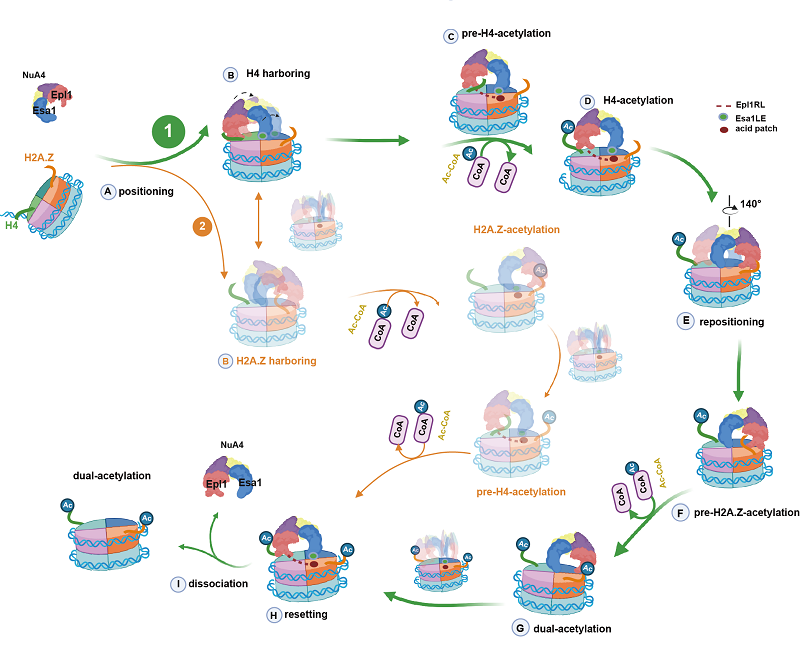
Schematic diagram of the process of pNuA4 acetylating different substrates on the H2A.Z-containing nucleosome (Image by ZHU Ping's group)
